February 2025 | Product Receives Taiwan FDA Approval
February 1, 2025
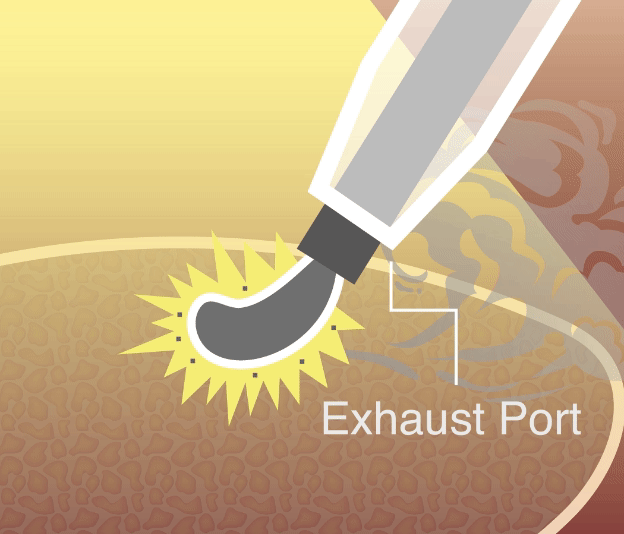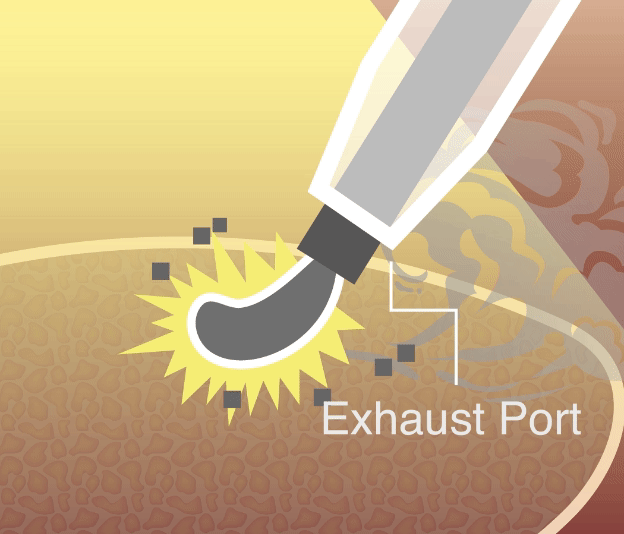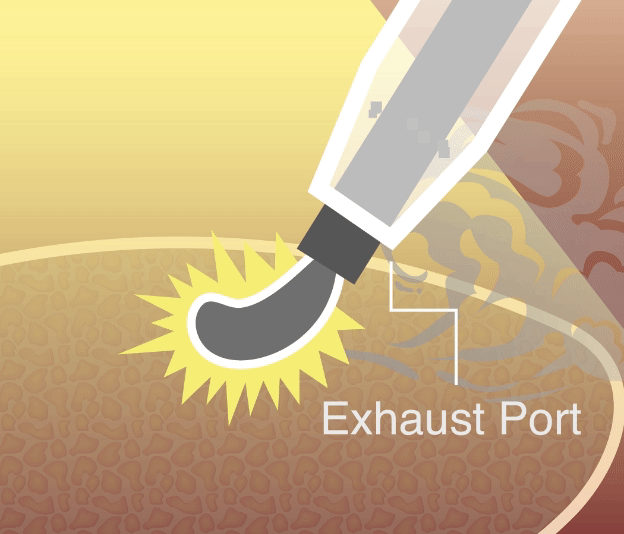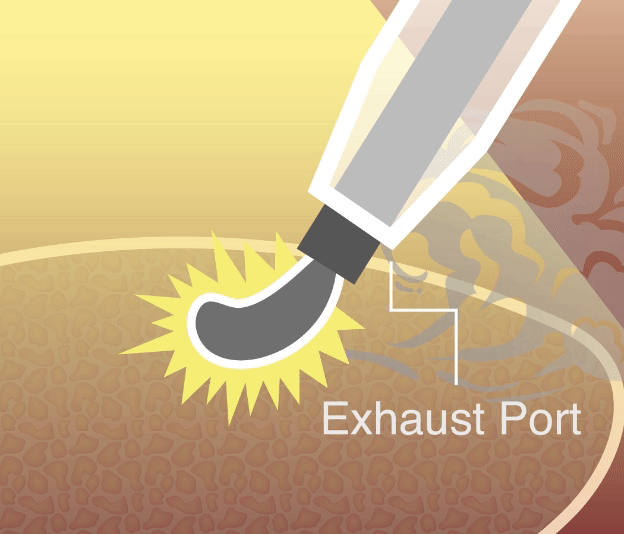
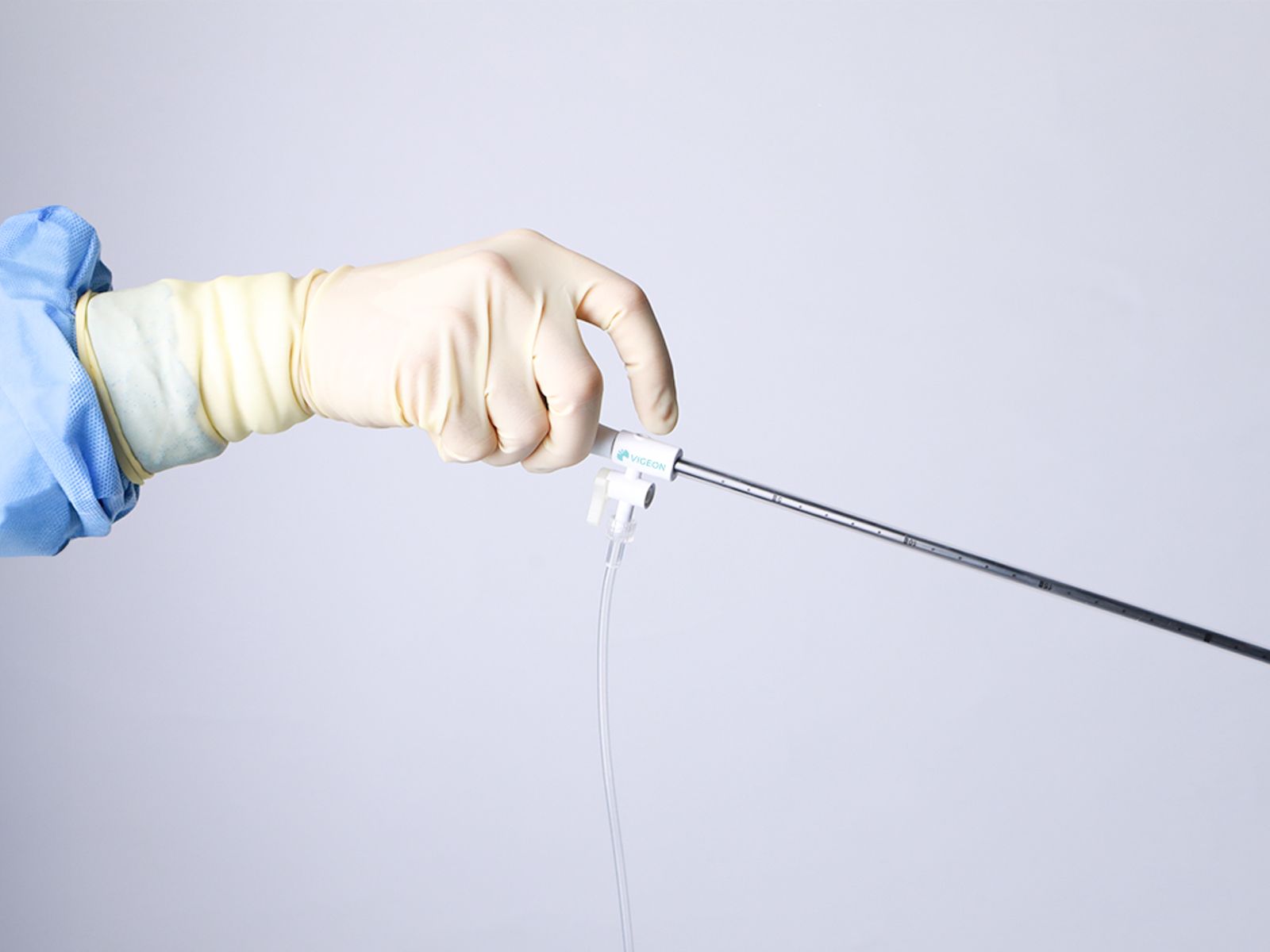
Our smoke evacuation device has officially received approval from the Taiwan Food and Drug Administration (TFDA). This milestone marks a major step forward in bringing safer, clearer laparoscopic solutions to clinical use in Taiwan.
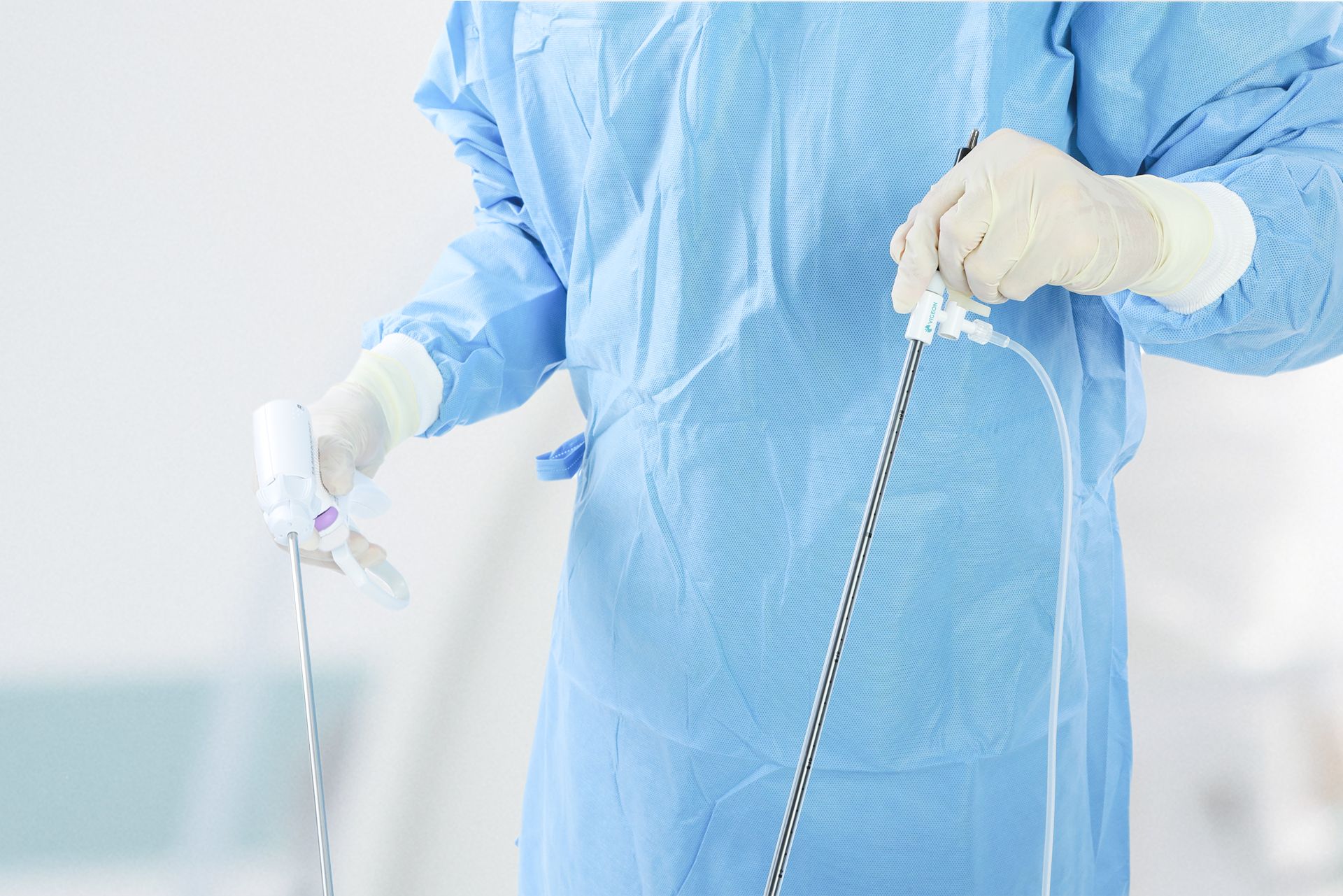

Vigeon participated in the 2025 Taiwan Healthcare+ Expo, showcasing its integrated surgical smoke evacuation solutions designed to improve visualization, safety, and workflow efficiency in minimally invasive surgery. The event provided an opportunity to engage with surgeons, hospital leaders, and industry partners on clinical needs, regulatory readiness, and future collaborations.