May 2025 | US FDA Approval Granted
May 1, 2025
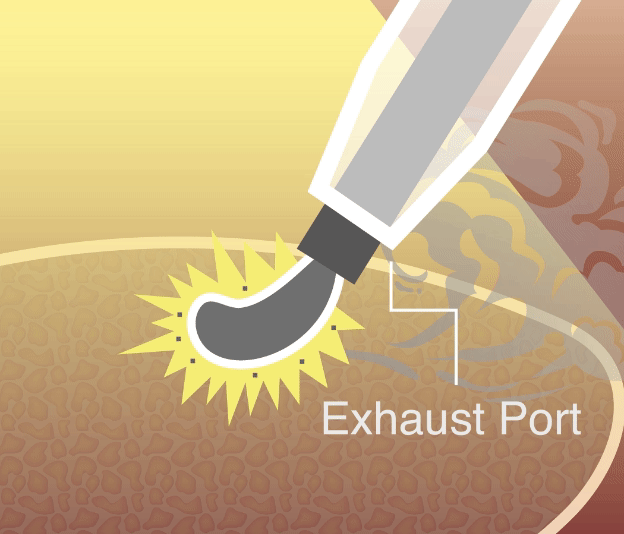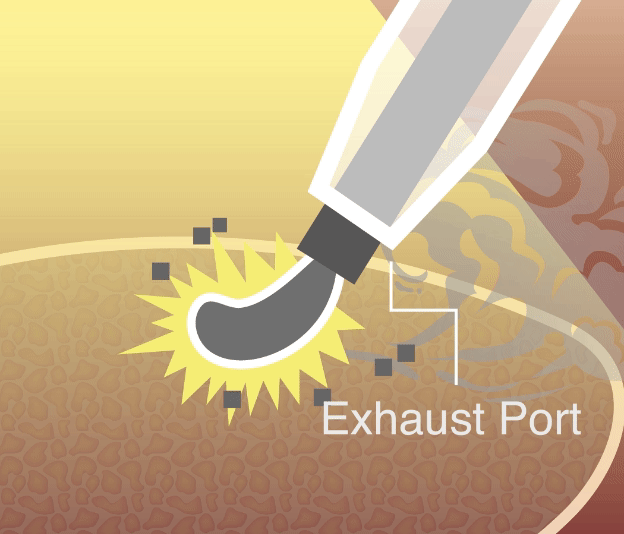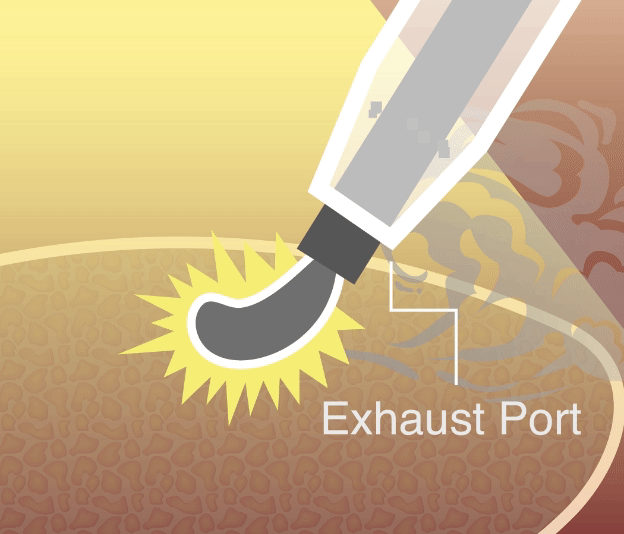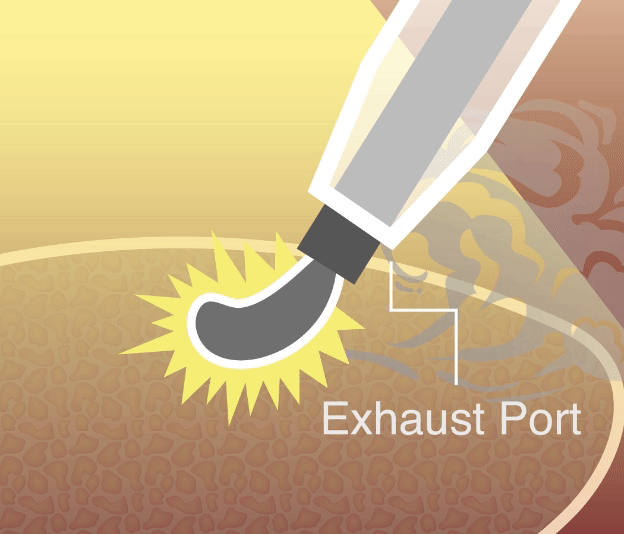
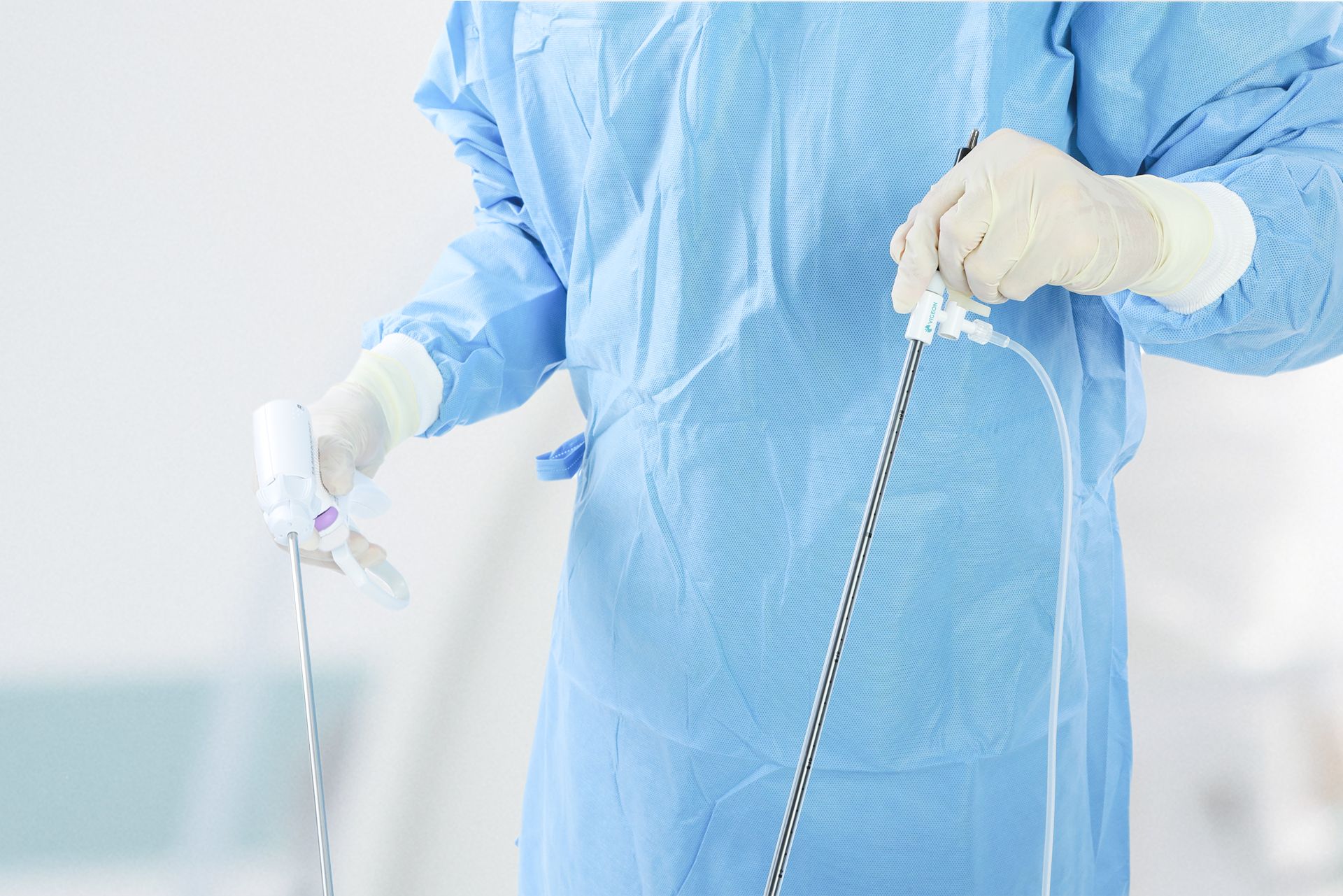
Happy to share that Vigeon’s smoke evacuation device received clearance from the United States Food and Drug Administration (FDA), validating its safety and efficacy in minimally invasive surgery!
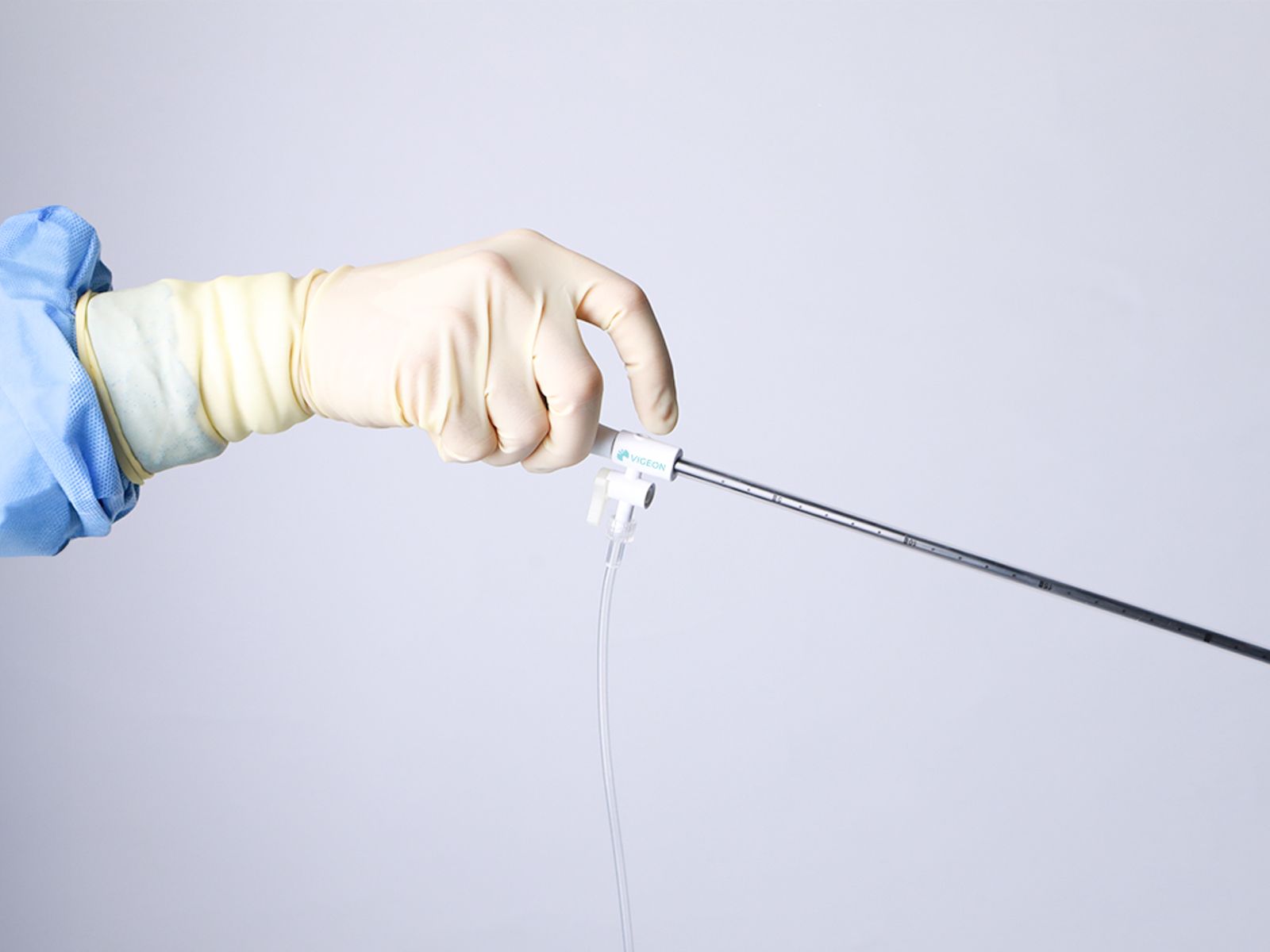

Vigeon participated in the 2025 Taiwan Healthcare+ Expo, showcasing its integrated surgical smoke evacuation solutions designed to improve visualization, safety, and workflow efficiency in minimally invasive surgery. The event provided an opportunity to engage with surgeons, hospital leaders, and industry partners on clinical needs, regulatory readiness, and future collaborations.